ENTEC in Horticulture - Nitrogen stabilisers in the soil
Nitrogen (N) management is a constant concern for Australian growers. Why? Because being able to consistently produce crops that are high yielding and possessing exceptional quality is very challenging. Unpredictable weather events can affect both crops & fertilisers adversely. In any farming system, fertiliser product selection is a key management option to reduce risk, achieve nutrient use efficiency, optimise production, and benefit quality.
Nitrogen stabilisers in the soil
Conrad Leeks and Rob Dwyer, Technical Agronomists – Incitec Pivot Fertilisers
Not all fertilisers perform equally. This is where ENTEC®, an ammonium stabiliser and Enhanced Efficiency Fertiliser, has a great fit in improving nutrition programs via the simple process of appropriate fertiliser selection. In this process, understanding the N cycle and losses within the cycle is vital.
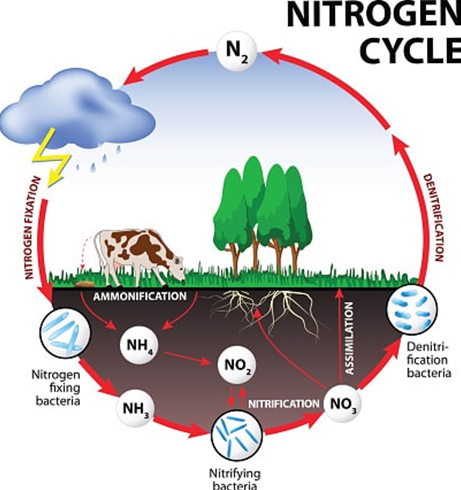
Figure 1: The Nitrogen cycle.
Nitrogen Sources
Soil N supply from organic matter
Mineralisation of soil organic matter is a significant contributor to crop N supply. Mineralisation involves soil microorganisms transforming the ‘non-plant available’ organically-bound N into ‘plant available’ mineral N. Any nitrogen associated with the likes of crop residues, fallow crops or composts, also enters the pool of organically-bound N.
Soil N supply from fertilisers
Soil testing is important in horticulture. Testing tells the difference between what ‘soil fertility’ can provide crops naturally, compared to the amount of nutrients crops require to flourish. The type and amount of nutrient differences between these ‘crop required’ & ‘soil fertility’ levels can be applied as fertilisers.
Nitrogen Losses
Immobilisation
Just as N is subject to biological transformation to be made ‘plant-available’ under certain soil conditions, a similar biological transformation can also tie-up nutrients. This is known as immobilisation, and it is considered to be a short to medium term N loss pathway. Over time, this N may become plant available again.
Nitrogen conversion chemistry - nitrification and leaching
During the process of nitrification, nitrogen is converted by Nitrosomonas soil bacteria from Ammonium (NH4+) into Nitrite (NO2-) and then into Nitrate (NO3-) by another group of soil bacteria, Nitrobacter:
NITRIFICATION
Ammonium (NH4+) ------------------ Nitrite (NO2-) --------------- Nitrate (NO3-)
Nitrosomonas Nitrobacter
In terms of loss pathways, the issue with NO3- is that it can be lost via both leaching and denitrification. Leaching takes nitrate through the soil & out of the root zone. Denitrification turns nitrate into gasses which are then lost from the soil to the atmosphere.
The reason NO3- leaching occurs is due to its negative charge, i.e. soil colloids and soil organic matter possess a negative charge, hence two negatives cannot attract. So as water moves through lighter soils, NO3- moves with it. There is a fine balance at play here – between water & NO3-. We need to regulary irrigate, to keep the crops productive. Then rainfall events occur also. However, every time water moves through the soil, NO3- is also moving with it. If nitrate moves beyond the root zone, crops can not access it to grow. Leaching is common in soils with a cation exchange capacity (CEC) of <4 cmol(+)/kg.
Denitrification occurs due to the microbes in soil, as they start running out of oxygen in wet to saturated conditions (i.e. 60% or greater water filled pore space). This can be worsened by poorly structured or compacted soils, where high soil organic matter levels create high soil microbe populations, with over irrigation or when experiencing significant rainfall events. Under these conditions, the microbes use NO3- as an oxygen supply. The conversion is as follows:
NO3- to NO2- to NO to N2O to N2
(Nitrate) (Nitrite) (Nitric Oxide) (Nitrous oxide*) (Di-nitrogen)
*A potent green house gas, 298 time worse than carbon dioxide (CO2).
Why ENTEC®?
ENTEC is an effective fertiliser product to apply to your crops as it slows the conversion rate of ammonium to nitrate. Ammonium leaches very little and cannot denitrify at all. This is the opposite to nitrate, which is prone to both leaching and denitrification. Crops can still take up N when it is in the NH4+ form, however some crops actually prefer ammonium N.
In Victorian trials, ENTEC Nitrophoska was shown to reduce the average nitrous oxide N2O emission flux by 69 to 100% compared to traditional fertilisers (Melville et al. 2012).
This means ENTEC keeps more nitrogen where you & your crop want it most – available and in the soil.
|
ENTEC® - Horticulture Crop Responses (statitically significant), Australia |
|||||||
|
Crop |
Location |
Product/s |
ENTEC |
Conventional |
Percentage Yield |
Statistics |
|
|
Potato |
Atherton |
Entec CK-800 v CK-800 |
39.25 t/ha |
34.95 t/ha |
12.3% |
LSD 3.79 |
|
|
Potato |
Atherton |
Entec Nitrophoska v Nitrophoska |
36.55 t/ha |
32.10 t/ha |
13.9% |
LSD 3.79 |
|
|
Potato |
Pinnaroo, 2016 |
Entec Nitrophoska v Nitrophoska |
73.05 t/ha |
59.65 t/ha |
22.5% |
LSD 10.55 |
|
|
Broccoli |
Boneo, 2012 |
Entec Nitrophoska v Nitrophoska |
4.98 kg/plot |
3.23 kg/plot |
58.6% |
LSD 1.29 |
|
|
Broccoli |
Boneo, 2012 |
Entec Nitrophoska v Nitrophoska |
5.55 kg/plot |
3.14 kg/plot |
76.8% |
LSD 1.29 |
|
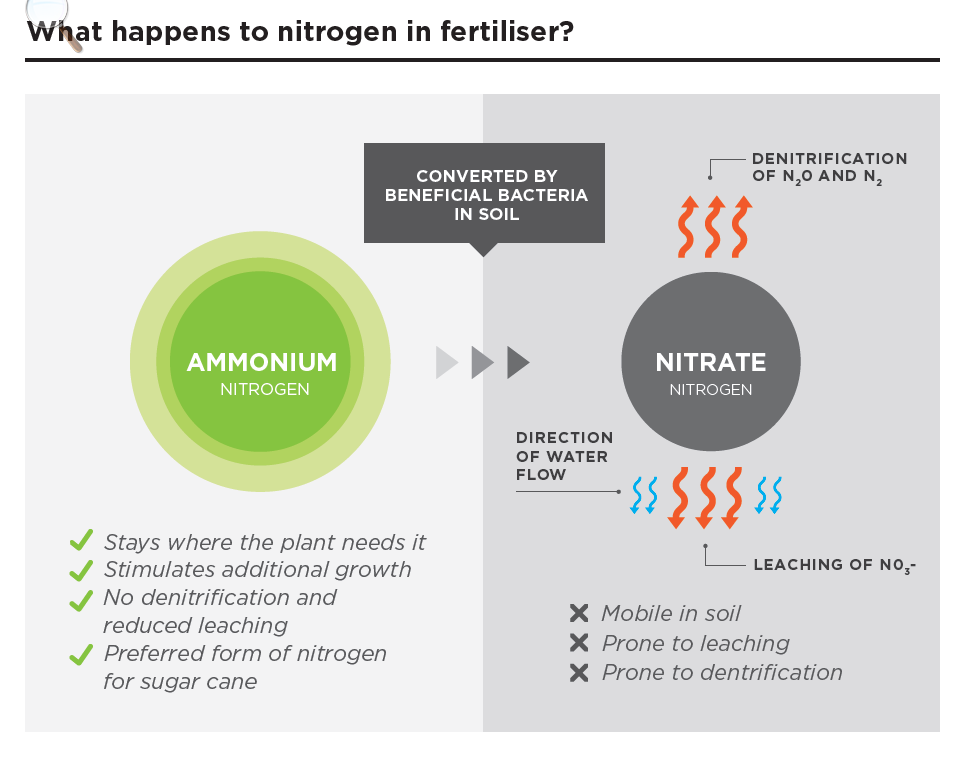
Improving N use efficiency
One way of increasing N use efficiency is by using Ammonium Stabilisers.
Ammonium stabilisers work by inhibiting the Nitrosomonas group of soil bacteria (‘bacteriastatic’ action), which really means N is kept in the top soil as NH4+ for a longer period of time. By inhibiting those bacteria, the NH4+ form of N remains as a positive charge, and therefore, will be not readily leach through the profile and has a zero denitrification loss potential.
During early establishment, the immediate need for N by transplants or direct seeded crops is relatively low, and thus nitrate N losses caused by leaching and denitrification may be especially high – under the wrong conditions. When applying a base fertiliser, ENTEC helps to reduce these nitrogen losses by keeping the N in the more stable and still plant-available ammonium form. As the crop becomes established, there is N available in the root zone for crop uptake as the N has been protected against leaching & denitrification losses.
Case study
In a situation where overhead sprinklers are used, stabilising NH4+ is of great benefit. For example, in a broccoli patch where there is crop residue present, wet conditions (due to overhead irrigation), compacted soil and warm temperatures, using ENTEC reduced N from being lost through both leaching & denitrification.
ENTEC has been shown to be effective in the soil from 4 – 10 weeks. This helps the broccoli plant by reducing NO3- leaching. Nitrates are moved into deeper soil layers, and hence are no longer available to plants. This has been demonstrated in laboratory results in a moist alkaline vertosol soil at 250C, 97% of applied fertiliser had converted to nitrate after 14 days without ENTEC protection. When ENTEC was used, over 70% of the nitrogen was stabilised as ammonium after 50 days (Suter et al. 2008).
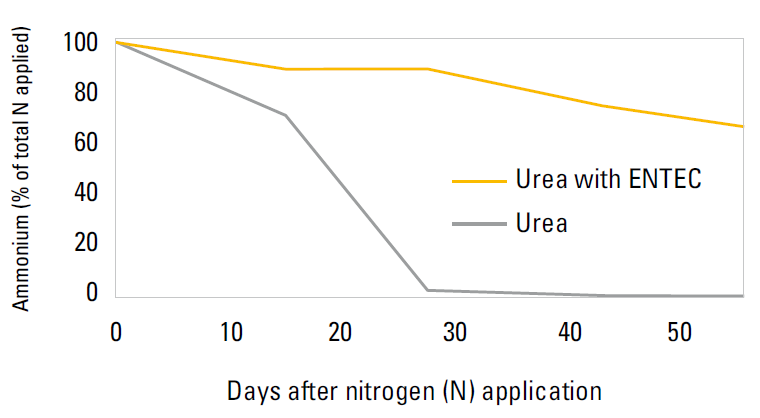
Flexibility
Using NH4+ stabilisers ensures flexibility regarding timing of application. Fertiliser application can be applied early in crop development, without compromising yield N loss potentials. Potentially fewer fertiliser applications can be required when using an NH4+ stabiliser, with more N being protected and ‘banked’ in the topsoil. Melville et al. (2012) demonstrated that one fertiliser application could be dropped in Victoria due to enhanced N use efficiency.
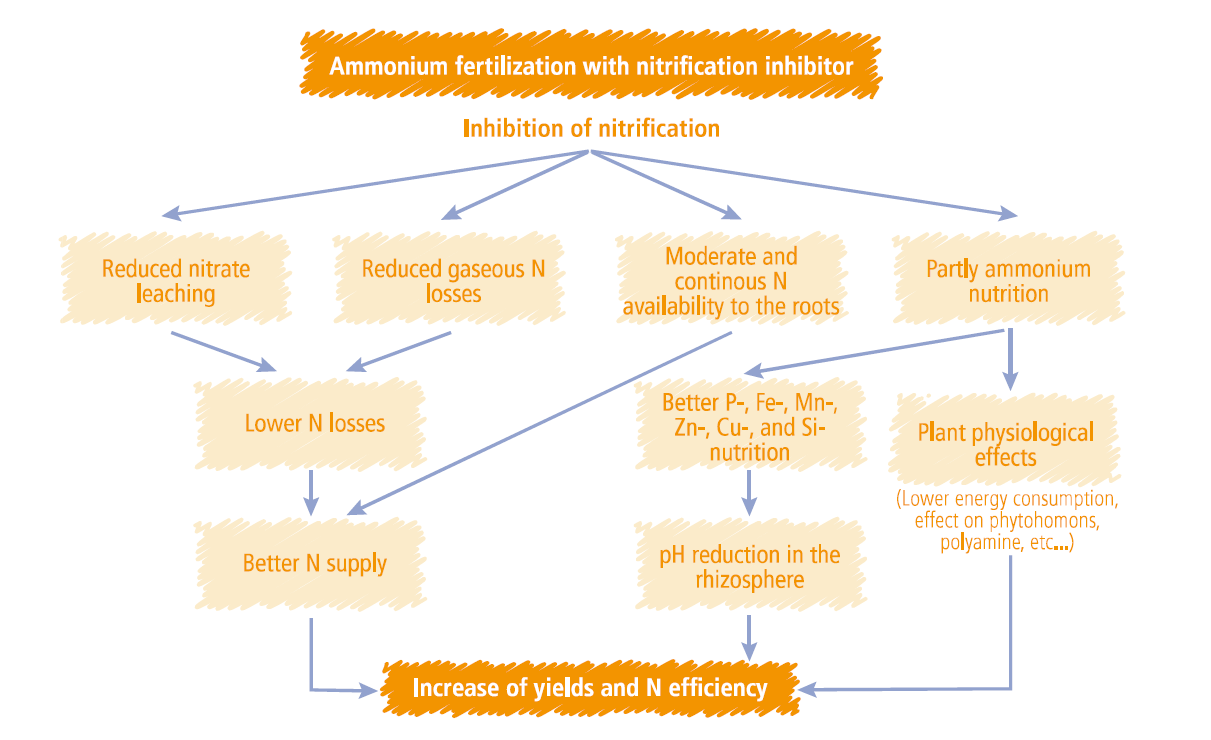
There is more to ENTEC than just reducing leaching & denitrification:
Crop physiological benefits
- Plants may spend less energy on NH4+ uptake than on NO3– uptake (Ullrich 1992; Wang et al. 1994), especially at a high rate of NH4+ supply (Glass et al. 1997).
- Ammonium is directly incorporated into amino acids and proteins. The energy saved in comparison to conventional nitrate nutrition can be used for other metabolic processes. Trenkle, 2010).
- Ammonium can be directly used for N adsorption (Pasda et al. 2001), whereas nitrate has to undergo reduction at a high energy cost to the plant prior to N uptake.
- Ammonium will be taken up by the plants rather than Nitrates when NH4+ is supplied to a crop together with NH4+ stabilisers Pasda et al. (2001).
- Ammonium has been established in various studies that it has a positive effect on the synthesis of polyamines, cytokinins and gibberellins. Klein et al. (1979), as well as Gerendás and Sattelmacher (1995), found higher concentrations of the polyamines putrescine and spermine when plants were supplied with NH4+ instead of NO3–. It is known that polyamines function as secondary messengers for flower induction. Better tillering and growth of tillers in cereals, and a higher number of fruits in fruit-bearing crops as the result of NH4+ nutrition, are seen in connection with the increased cytokinin metabolism. Smiciklas and Below (1992) and Wang and Below (1996) detected higher cytokinin contents in maize and wheat, when the plants received NH4+ instead of NO3-.
- Significantly higher concentrations of gibberellin have been found in xylem sap with NH4+ and mixed NH4+/ NO3- nutrition, compared to NO3- nutrition only (Ali et al. 1998).
- Ammonium stabilisers can decrease the soil pH in the rhizosphere (Trolldenier 1981; Thomson et al. 1993). The localised lower pH will improve the availability of other nutrients, especially micronutrients, for plant uptake.
- Application of NH4+ combined with P can significantly improve maize growth and nutrient use efficiency during early growth stages by stimulating root proliferation and rhizosphere acidification (Jing et al. 2010).
- On farm, this could be achieved by using Nitrophoska/ENTEC or blends containing MAP/ENTEC or DAP/eNpower.
- Partial NH4+ nutrition can decrease the NO3- concentration of the crop (Slangen and Kerkhoff 1984; Venter 1984; Müller and Wedler 1987; Burghardt and Ellering 1988; Hähndel et al. 1994), but not its N uptake (Buerkert et al. 1995). As a result, trials have shown that plants are darker in colour and more upright when using ammonium stabiliser treated fertilisers.
Advantages of Ammonium nutrition
- Low energy consumption for nutrient uptake, increased N uptake even when low root zone temperature is experienced;
- Low energy consumption for N assimilation in the plant, because Ammonium is already reduced & available for use by the crop;
- Increased synthesis of the phytohormones like cytokinin, and larger content of polyamines.
For more information on making the most of your fertiliser programs, please contact us on conrad.leeks@incitecpivot.com.au / 0466 664 026 or rob.dwyer@incitecpivot.com.au / 0428 111 471.
References:- Hähndel R, Lang H, Hermann P (1994) Wirkung von Ammoniumstabilisierten Düngern auf Ertrag und Qualität von Gemüse. Agribiol Res 47:101–108.
- Buerkert B, Horlacher D, Marschner H (1995) Time course of nitrogen in soil solution and nitrogen uptake in maize plants as affected by form of application and application time of fertilizer nitrogen. J Agron Crop Sci 174:325–336.
- Burghardt H, Ellering K (1988) Beeinflussung des Nitratgehaltes von Spinat durch unterschiedliche Kulturbedingungen. Gartenbauwissenschaft 53:201–205.
- Müller H, Wedler A (1987) Ein Beitrag zum Einsatz des Nitrifkationshemmstoff Dicyandiamid (DCD) zur Verminderung des Nitratgehaltes in Gemüse. Landwirtsch Forsch 40:78–87.
- Venter F (1984) Nitrate contents of head lettuce as influenced by nitrificides. Acta Hortic 163:231–236.
- Slangen JHG, Kerkhoff P (1984) Nitrification inhibitors in agriculture and horticulture: a literature review. Fert Res 5:1–76.
- Ali IA, Kafkafi U, Ymaguchi I, Sugimoto Y, Inanaga S (1998) Response of oilseed rape plant to low root temperature and nitrate: ammonium ratios. J Plant Nutr 21:1463–1481.
-
Gerendás J, Sattelmacher B (1995) Einfluß des Ammoniumangebotes auf Wachstum, Mineralstoff- und Polyamingehalt.
Junger Maispflanzen. Z Pflanzenernaehr Bodenkd 158:299–305. - Klein, H, Priebe, A., & Jager, H. (1979) Putrescine and Spermidine in Peas: Effects on Nitrogen Source and Potassium Supply. Physiologia Plantarum, 45: 497-499.
- Melville et al. (2012) Carbon and Sustainability. A demonstration on vegetable properties across Australia.
- Pasda, G., Handel, R. and Zerulla, W (2001) Effects of fertilizers with the new nitrogen inhibitor DMPP (3,4-dimethlypyrazole phosphate) on yield and quality of agricultural and horticultural crops. Biological Fertility Soils, 34, 85-97Jing et al. (2010) Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crops Research. 119. 355-364.
- Ulrich, W. (1992) Transport of nitrate and ammonium through plant membranes. In: Mengel K. Pilbeam D. (eds) Nitrogen metabolism of plants. Oxford Science, Clarendon press, Oxford, pp 121-137.
-
Smiciklas KD, Below FE (1992) Role of cytokinin in enhanced productivity of maize supplied with NH4+ and NO3-. Plant Soil. 142:307–313.
‘Ammonium uptake by rice roots’ Wang, M., Glass, A., Shaff, J. and Kochian, L. (1994) III. Ectrophysiology. Plant Physiology, 104, 899-906. - Wang X, Below FE (1996) Cytokinins enhanced growth and tillering of wheat induced by mixed nitrogen source. Crop Sci. 36:121–126.Trolldenier G (1981) Influence of soil moisture, soil acidity and nitrogen source on take-all of wheat. Phytopathol Z 102: 163–177.


